Updated ‘New Protocol’ Process
Streamlined and improved the process for creating a new protocol:
- Protocol Template
- Copy selected sections from an existing protocol.
- Better than “Save as”: pick and choose what information to copy, eliminating the need to review and update all fields for the current year.
- Custom Settings
- Protocol Checklist provides overview of study settings prior to protocol creation.
- New Study Rules and SE Lists sections in settings to pre-load these important protocol features.
- These new selections can be saved in the saved “Settings Set” defaults for future re-use.
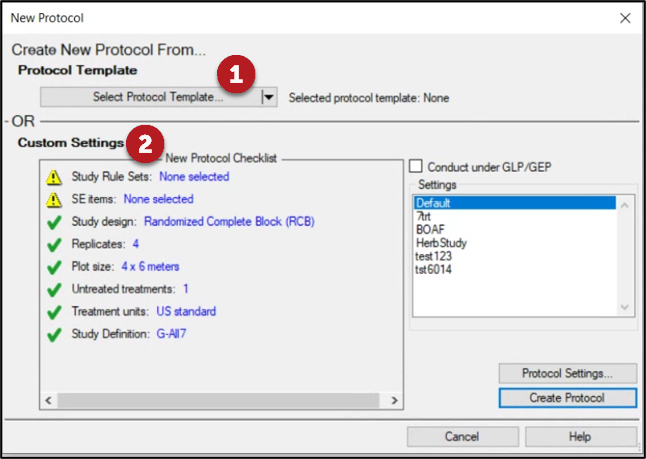
New Protocol Checklist
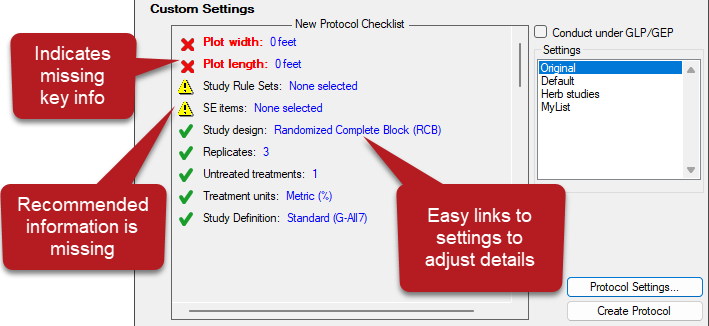
- Interactive overview of study settings for protocol creation
- Red X indicates key information that is missing; Yellow warning indicates recommended (but not required) information is missing.
Protocol Planning
Limit who can view individual rows on the Trial Location table with new fields (GDM ID, Company ID, Company Name):
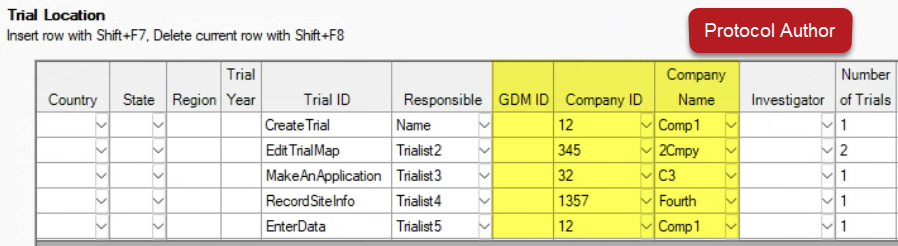
Trialists only see rows from their own company (via GDM ID or Company ID) when viewing the protocol:
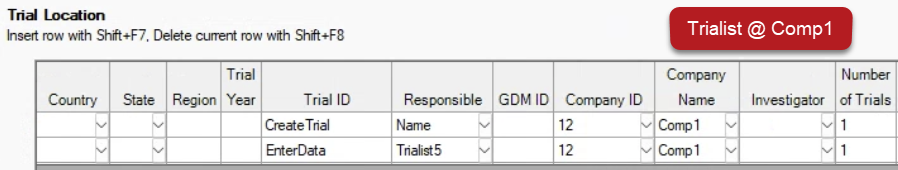
Tip: Obtain the GDM ID/Company ID from contractors by having them select Send License Details found on Help > Profile.
For more information on the Trial Location table, see Planning Trial Locations in a Protocol.
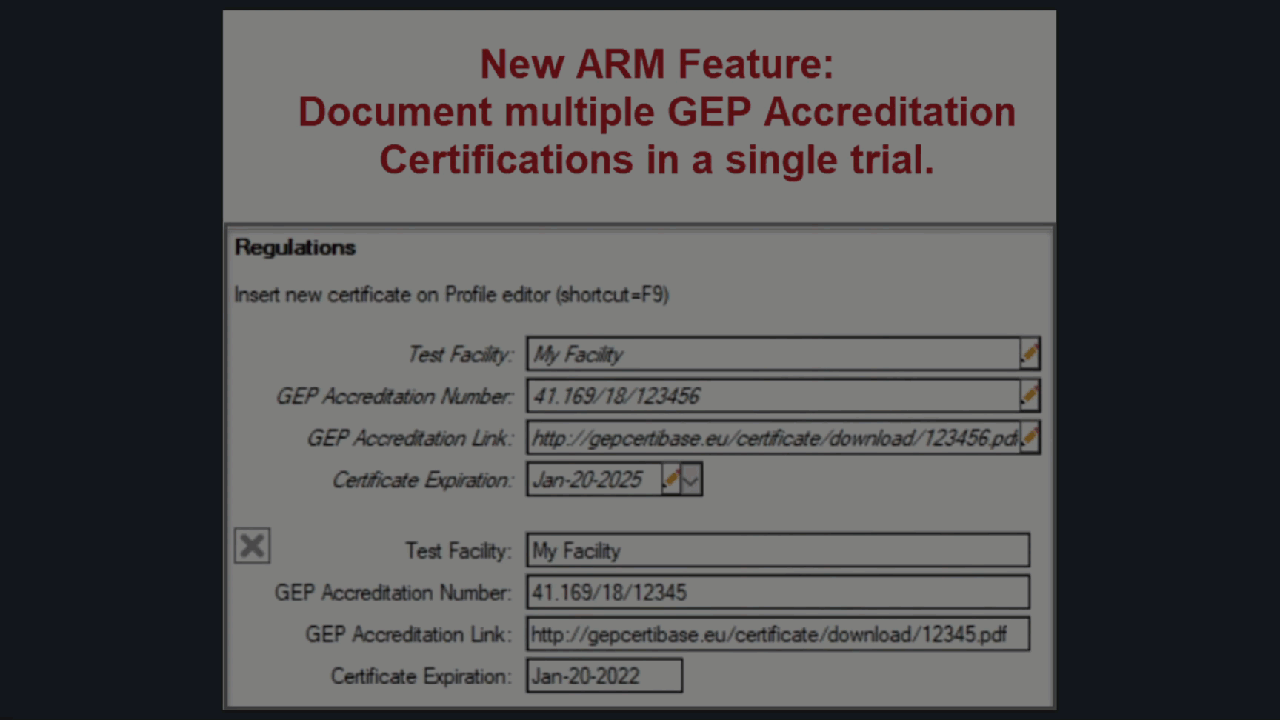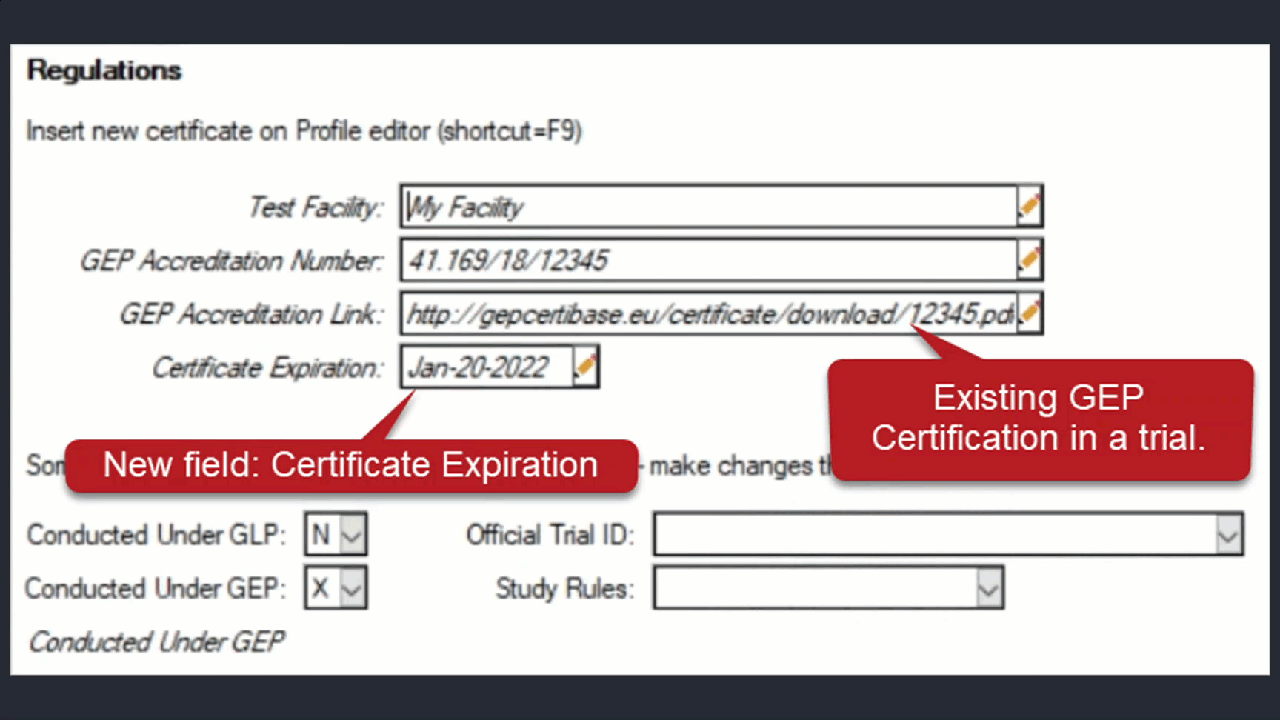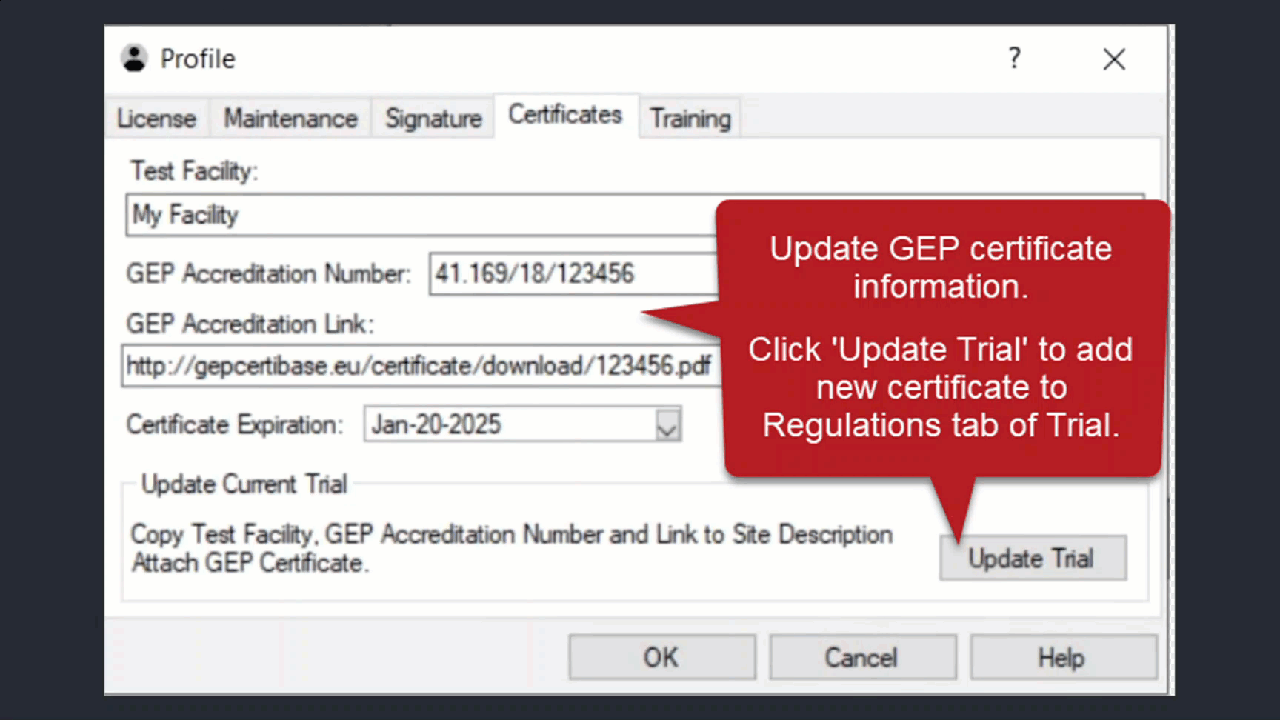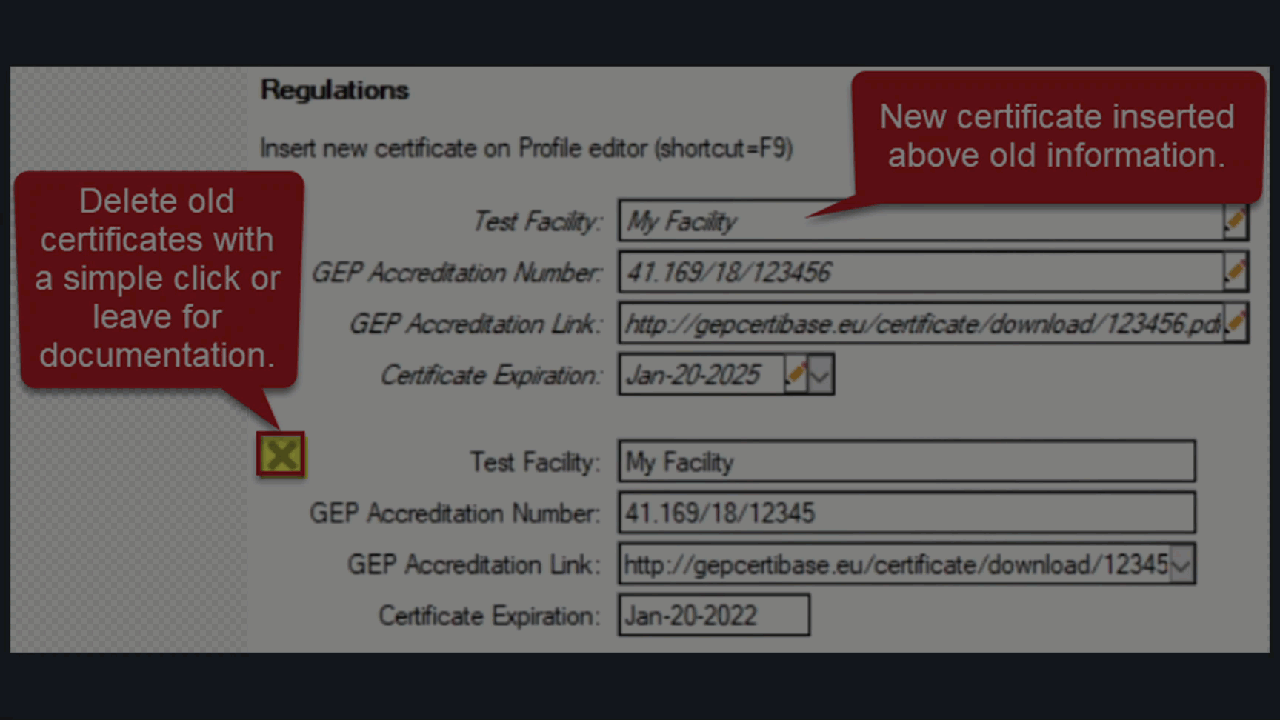
Document multiple GEP Accreditation Certificates
ARM now supports multiple GEP Certificates for a single trial.
Use Profile > Certificates > Update Trial to add the new/updated certificate to the current trial.
complete change log available here
